Reading about biochemistry, organic chemistry, or any other similar discipline will necessarily include the discussion of carbon-based compounds. Carbon atoms, as stated by Mason et al. (2014), are the framework of biological molecules.
WHAT IS A CARBON ATOM?
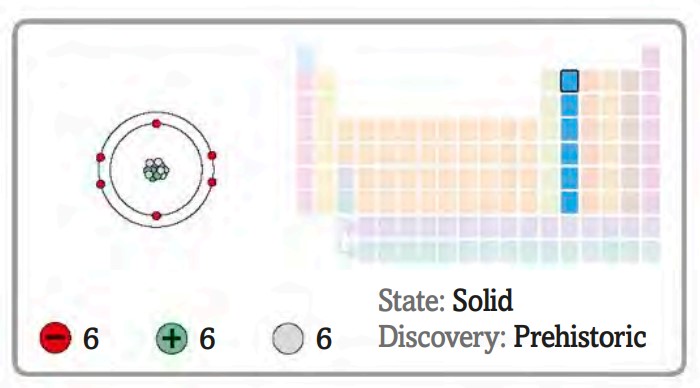
Carbon is an element that can be found in the periodic table (group 14, period 2). It has the largest number of compounds of any element, with more than nine million known (Challoner and Jackson 2017). It is the fourth most common element in the Universe, and each carbon atom can bond to four others. It has 6 electrons, 2 in its first shell and 4 in its outermost shell, having a 1s22s22px12py12pz0 structure.
CARBON IN BIOLOGICAL SYSTEMS
The framework of biological molecules is mainly comprised of carbon atoms bonded to other carbon atoms or atoms of oxygen, nitrogen, sulfur, phosphorus, or hydrogen (Mason et al. 2014). In other words, almost all the molecules a cell makes are composed of carbon atoms bonded to one another and atoms of other elements (Taylor et al. 2018).
This element is capable of forming large and complex molecules, building the structures, and carrying out the functions required for life (Taylor et al. 2018). Carbon-based molecules are known as organic compounds, and most of the time they contain hydrogen atoms in addition to carbon atoms. Organic compounds are the subject matter of sciences like organic chemistry, biochemistry, and biology in general.
Carbon atoms are great to form large molecules like the ones required for life to exist because, according to the octet rule, an atom will behave as if it were a noble gas even if its outermost shell is not full as long as it has at least 8 valence electrons.
Constable & Housecroft (2006) define the octet rule as “an atom is obeying the octet rule when it gains, loses, or shares electrons to give an outer shell containing eight electrons with the configuration ns2np6.”
In the case of a carbon atom, it would be 2s2,2p6.
A carbon atom has 4 electrons in its outermost shell that can hold up to 8. Hence, it completes its outer shell by sharing electrons with other atoms in four bonds. Because carbon atoms can form up to four covalent bonds, molecules containing carbon can form straight chains, branches, or even rings, balls, tubes, and coils (Mason et al. 2014).


Methane is one of the simplest organic molecules, which is made of one carbon atom and four hydrogen atoms, generally written as CH4 (Taylor et al. 2018). In more complex organic compounds with more than one carbon atom, each one is a connecting point from which a molecule can branch in up to four directions.
Carbon atoms can form double bonds in organic molecules, resulting in varying shapes (Taylor et al. 2018). For instance, butene is an organic compound with a double bond between two carbon elements, and it can come in two different forms (1-Butene and 2-Butene), each one with a double bond in different places and hence changing the entire molecule’s shape and the location of some of its hydrogen atoms (see picture above).
In general, carbon chains form the backbone of most organic molecules, and they are called carbon skeletons as well. They may differ in several forms (see picture above).
Two types of organic compounds can have the same molecular formula, yet differ in shape, which is the case of molecules like Butane and Isobutane, and 1-Butene and 2-Butene. Whenever a compound shares the same formula with another one, but both differ in their structural arrangement, they are called isomers (Mason et al. 2014, Taylor et al. 2018).
REFERENCES
Challoner, J. & Jackson, T. (2017). The periodic table book. A visual encyclopedia of the elements
Constable, E. & Housecroft, C. (2006). Chemistry. 3rd edition
Mason et al. (2014). Biology. 11th edition
Taylor et al. (2018). Campbell biology concepts & connections. 9th edition
